Sexually Transmitted Diseases and Miscellaneous Pelvic Infections
Authors
INTRODUCTION
The human female genital tract affords the medical practitioner a rate challenge of his diagnostic acumen and command of the healing arts. The vagina is an object of close interpersonal contact, including sexual intercourse; therefore, a variety of organisms may be spread from person to person by this route. In addition, since the vagina is in close proximity to the gastrointestinal tract, certain enteric pathogens may clinically manifest as gynecologic disease. Because the female genital tract is virtually a direct conduit between the outside world and the peritoneal cavity, many of the infections thus contracted may progress beyond the confines of the female genitalia to cause generalized intraperitoneal and systemic disease.
Classically described “venereal diseases,” including gonorrhea, syphilis, chancroid, lymphogranuloma venereum, and granuloma inguinale, are now termed sexually transmitted diseases (STD). They have been joined by an array of bacterial, viral, fungal, and even animal pathogens as listed below:
Sexually Transmitted Diseases
Gonorrhea
Syphilis
Herpes simplex
Trichomoniasis
Venereal warts
Gardnerella vaginitis
Candidiasis
Pediculosis pubis
Urogenital chlamydial infections
Lymphogranuloma venereum
Genital mycoplasmas
Chancroid
Granuloma inguinale
Hepatitis B
Cytomegalovirus
Scabies
Group B streptococcus
In addition, gastrointestinal inhabitants and acid-fast bacilli may infect the female pelvis, as illustrated below:
Miscellaneous Pelvic Infections
Actinomycosis
Listeriosis
Shigellosis
Schistosomiasis
Amebiasis
Blastomycosis
Coccidioidomycosis
Enterobiasis
Tuberculosis
Syndromes caused by these various organisms are vulvitis, vaginitis, urethritis, cervicitis, endometritis, and salpingitis. Since separate pathogens may produce similar, even identical, clinical illnesses, this chapter is organized by organism, rather than by disease entity. Several of the microbes are considered in depth in other chapters and are therefore excluded here.
CHLAMYDIA TRACHOMATIS
The causative organism of endemic trachoma, inclusion conjunctivitis, lymphogranuloma venereum, urethritis, and cervicitis has been the subject of much medical literature in recent years. Only since 1965 has there been a relatively reliable, easily performed isolation technique, namely, tissue culture.1 Since that laboratory breakthrough, profuse data have been generated about human disease caused by Chlamydia trachomatis, as well as human and avian disease caused by C. psittaci, the agent of psittacosis. Because of the impact of C. trachomatis as a cause of pelvic inflammatory disease (PID), perhaps the largest portion of recent literature has concerned the urogenital chlamydial syndromes.
Bacteriology
The chlamydiae are obligate intracellular parasites of eukaryotic cells because of their inability to synthesize adenosine triphosphate (ATP). At one time they were considered viruses and given various generic names, including Bedsonia, Miyagawanella, and TRIC agent (trachoma-inclusion conjunctivitis). These organisms are now considered prokaryotic in nature--a special type of bacteria. They are known to have both deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), have a cell wall that stains negatively on Gram stain, divide by binary fission, and be susceptible to antibiotics.
The order Chlamydiales consists of one family, Chlamydiaceae, which is made up of only one genus, Chlamydia. The two species, C. trachomatis and C. psittaci, are differentiated by sensitivity to sulfonamides and production of glycogen (C. trachomatis only).2
C. trachomatis may be separated into multiple serotypes by microimmunofluorescence techniques. Serotypes A, B, Ba, and C cause the syndrome of endemic trachoma. Serotypes D, E, F, G, H, I, J, and K cause neonatal conjunctivitis, neonatal pneumonitis, and a variety of sexually transmitted syndromes (cervicitis, urethritis, epididymitis). Finally, serotypes L1, L2, and L3 cause the venereal disease lymphogranuloma venereum (LGV).3
Although examination of Giemsa-stained scrapings of clinical materials was used successfully for diagnostic purposes in cases of chlamydial conjunctivitis, cell-culture methods are necessary in the urogenital syndromes. Microimmunofluorescent antibody titer studies may also be used.4 However, this method is difficult because the disease may be chronic, so the traditional “acute-and-convalescent” method of titers may be undependable.
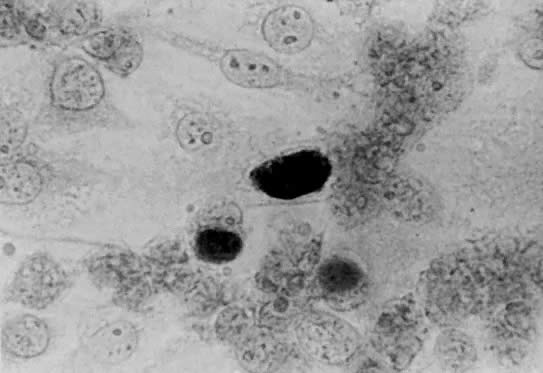
Both LGV and non-LGV strains of chlamydia may be grown in McCoy cells, a heterophile mouse cell-culture line. These cells are first inhibited by irradiation or with 5-iodo-2-deoxyuridine, permitting the chlamydia to more easily compete for cell nutrients. This allows easier recognition of intracytoplasmic inclusions after staining with iodine (Fig. 1).5
|
Since the diagnosis of conjunctival chlamydial infection was at one time made primarily on the basis of cytologic scrapings, investigators have tried to correlate the Papanicolaou smear with urogenital infection, especially of the cervix. Some researchers believe that intracytoplasmic coccoid and inclusion bodies in dysplastic exfoliated cells :are characteristic of chlamydial infection.6 Others have contradicted this claim, finding no regular morphologic features in chlamydia-infected specimens except for nonspecific inflammatory and dysplastic changes.7 The question remains unsettled, although individual cytologists are occasionally confident enough to read a routine smear as “changes suggestive of chlamydial infection.”
Clinical Features
C. trachomatis is causative, or at least implicated, in a variety of human disease syndromes, which are listed below:
Nongonococcal urethritis
Postgonococcal urethritis
Acute urethral syndrome
Acute salpingitis
Chronic salpingitis
Postabortal pelvic infection
Post-cesarean section infection
Endemic trachoma
Neonatal conjunctivitis
Neonatal pneumonia
Abortion
Stillbirth
Prematurity
“Late” postpartum endometritis
Reiter's syndrome has been associated with chlamydial urogenital infection.8 Chlamydial myocarditis has been described,9 as has endocarditis.10 The majority of investigation now concerns urogenital chlamydial syndromes.
Initially it was noted that a “sterile” form of acute mucopurulent conjunctivitis, similar to trachoma in that inclusions were noted in the cellular secretions, occurred in newborns. Interestingly, some parents exhibited those same inclusions in cells shed from areas of urethritis or cervicitis.11 In recent years, a distinct pneumonia syndrome in children under 6 months of age has been associated with colonization by chlamydia in the nasopharynx and trachea.12 These discoveries led to investigation of adult genital carriage of the organism, presumably as a reservoir for neonatal inoculation.
It is now fairly well accepted that C. trachomatis is one of the most frequently isolated sexually transmitted pathogens. C. trachomatis was first implicated in male nongonococcal urethritis (NGU) in 1966.13 By 1975, this relationship was firmly established by finding chlamydia in 42% of men with NGU, as opposed to 19% of men with gonococcal urethritis.14 Now C. trachomatis is recognized as a major cause of the female urethral syndrome (sterile pyuria and dysuria).15, 16 C. trachomatis and Neisseria gonorrhoeae have been found to coexist in more than 50% of patients with gonorrhea. Therefore, treatment of the gonorrhea with drugs unsuitable for treatment of chlamydia may result in “postgonococcal” urethritis.17
C. trachomatis is one of the most common causes of acute salpingitis, especially in Europe.18, 19, 20 Chlamydial salpingitis differs clinically from gonococcal disease in that the course is more protracted and symptoms more vague, although severe inflammatory changes occur in the internal female genitalia.21 Associated perihepatitis, the Fitz-Hugh--Curtis syndrome, has been reported in cases of chlamydial salpingitis.21, 22 The organism has subsequently been found in the peritoneal cavity of women undergoing tubal surgery to correct postinflammatory tubal infertility.23
The pregnant patient has recently become the object of attention in chlamydia research. Carrier rates of 2% to 23% have been reported.24, 25 Recent studies suggest that poor pregnancy outcome, such as abortion, stillbirth, and prematurity, may be up to ten times more frequent in pregnancies complicated by maternal chlamydial infection.26 Pelvic infection after elective abortion has been associated with C. trachomatis colonization.27 Colonized women delivered by cesarean section may subsequently suffer severe pelvic infection,27 and colonized women delivered vaginally are at risk for development of late (up to 6 weeks) postpartum endometritis due to C. trachomatis.28
The three serotypes of C. trachomatis that are responsible for LGV are inherently more invasive than non-LGV strains. The initial lesion on the genitalia is usually overlooked, and the organism ascends to the regional lymph nodes, causing a suppurative regional adenopathy with constitutional symptoms 2 to 6 weeks after initial exposure. The inguinal nodes are most often affected, whereas the hypogastric and iliac nodes may be involved in anorectal disease; obturator and iliac nodes may be affected after upper vaginal or cervical infection. The nodes are usually painful and may suppurate and become confluent and matted if left untreated, ultimately forming multiple draining fistulas. Sequelae include perirectal abscess, fistula in ano, and rectovaginal, rectovesical, and ischiorectal fistulas. Rectal strictures, keloid formation, and lymphedema are late complications.
The initial vesiculoulcerative lesion in LGV, if seen, is difficult to differentiate clinically from other ulcerative genital lesions. Early inguinal adenopathy must be differentiated from pyogenic adenopathy (secondary to distal infection due to staphylococci or streptococci), granuloma inguinale, secondary lues, chancroid, lymphoma, and miscellaneous infectious diseases such as bubonic plague, brucellosis, and cat-scratch disease.29
Diagnosis
The cornerstone of diagnosis of chlamydial infection is cell culture. Specimens collected by swab from the urethra and cervix should be immediately immersed in sucrose-phosphate transport medium and inoculated as soon as possible into McCoy cell monolayers.30 Refrigeration (not freezing) is preferable if inoculation is to be delayed. It should be emphasized that swabs of infected areas are necessary for reliable recovery of this organism; since C. trachomatis is a cell-associated pathogen, fluid cultures (e.g., culdocentesis fluid) are much less reliable than swab cultures (e.g., swab of fallopian tube), which are more apt to yield appropriately infected epithelial cells.
Even optimal laboratory facilities may take up to a week or more to report results, since this is a tissue culture procedure. In addition, tissue culture laboratories are not always available. Therefore syndromes associated with chlamydia may frequently be diagnosed either clinically or by less effective methods.
In patients with disease associated with chlamydia (e.g., salpingitis), failure to respond to therapy not directed against chlamydia (e.g., penicillin) should alert the physician to the possibility of chlamydia as the causative agent. Negative bacterial cultures (e.g., in urethritis) may also be a warning.
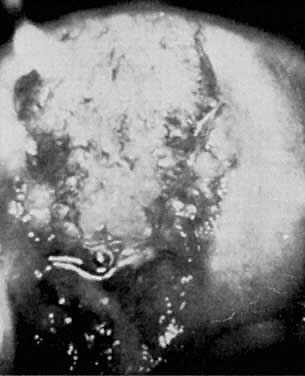
Chlamydial cervicitis has a distinctive presentation (Fig. 2). A follicular, exophytic cervicitis with mucopurulent endocervical discharge is classic.31, 32 This colposcopic appearance and a Papanicolaou smear showing inflammatory atypia or even dysplastic changes should suggest the possibility of chlamydial infection.
|
Serologic diagnosis of chlamydial infection by the acute-and-convalescent titers method is possible by microimmunofluorescent technique, although interpretation must be made with caution. The benefits of this method are that individual serotypes may be identified. Conversely, if the appropriate type-specific antibody is not included in the particular testing serum, seroconversion may be missed or inaccurate because of incomplete cross reactivity with the infecting serotype.
In general, a high index of suspicion and a thorough knowledge of the types of disease entities caused by C. trachomatis are the best weapons the clinician has against this organism.
Therapy
One of the early characteristics that led researchers to believe that C. trachomatis was not a virus was its sensitivity to antibiotics. In vitro studies have demonstrated C. trachomatis to be sensitive to the tetracyclines, erythromycin, sulfonamides, and rifampin.29, 33 Of interest to the obstetrician-gynecologist, however, are the reports that the popular new penicillin and cephalosporin drugs are inactive against this organism.34
The Centers for Disease Control in Atlanta, recommends treatment of proven chlamydial disease with tetracycline, 500 mg orally, four times/day, for at least 7 days. Alternatively, doxycycline, 100 mg twice a day, may be used. In patients with contraindications to tetracycline therapy, including pregnant women, erythromycin, 500 mg four times/day, is effective. If untoward gastrointestinal side-effects occur with this dose of erythromycin, the dosage may be halved (i.e., 250 mg four times/day), but the therapy must be extended to 14 days.35 Alternative therapy proven effective for urethritis includes a 10-day course of sulfonamides36 or 10 days of trimethoprim-sulfamethoxazole.37
Chlamydial conjunctivitis in the newborn may be prevented by erythromycin ointment ocular prophylaxis instead of the usual 1% silver nitrate solution; this regimen is accepted by the American Academy of Pediatrics38 and the National Society to Prevent Blindness.39 Topical therapy may not prevent nasopharyngeal infection or subsequent pneumonia.40 Established chlamydial newborn conjunctivitis or infant pneumonia should be treated with oral erythromycin syrup, 50 mg/kg/day in four divided doses, for at least 3 weeks.35
LGV is treated much the same as other C. trachomatis infections except for duration of therapy. Tetracycline, doxycycline, or erythromycin may be used but must be continued for 2 to 3 weeks. In addition, sulfamethoxazole, 1 g twice a day for 2 to 3 weeks, may be used. It is important that any buboes be aspirated through adjacent normal skin to prevent rupture; incision and drainage should be avoided, since it will delay healing. Any late sequelae, such as rectal stricture, must be dealt with appropriately after the acute infection is controlled.29, 35
GENITAL MYCOPLASMAS
An etiologic agent of bovine pleuropneumonia was first isolated in 1898 and termed the “pleuropneumonia organism.”41 Subsequent isolations of similar appearing organisms from several different animal species were termed “pleuropneumonia-like organisms” (PPLO). The first human myco-plasma isolation was from a Bartholin's gland abscess in 1937.42 However, the first clear association between mycoplasmas and human disease was made in 1961 when Eaton-agent pneumonia was linked to Mycoplasma pneumoniae infection in military recruits and later in school children.43
Recently, because of increased interest in STD syndromes spurred by C. trachomatis and the various viral agents, the genital mycoplasmas have received increasing attention in the obstetric and gynecologic literature.
Bacteriology
The mycoplasmas are a group of prokaryotic organisms that are appropriately thought of as bacteria with no cell wall. They are the smallest free-living organisms, distinct from viruses in that they grow in cell-free media and appear to divide by binary fission. The mycoplasmas are morphologically small, extremely pleomorphic, and gram-negative, growing mainly as facultative anaerobes.
Prokaryotic cells bound by a single membrane (i.e., no cell wall) are placed in the class Mollicutes, order Mycoplasmatales. The family Mycoplasmataceae contains those genera that require sterols for growth. The genera of interest are Mycoplasma, which metabolizes glucose or arginine, and to which belong the human pathogens M. pneumoniae and M. hominis, as well as several other species that are serologically distinguishable; and Ureaplasma, consisting of a single species that metabolizes urea, U. urealyticum, formerly termed “T-strain mycoplasma,” since its colonies are especially tiny on agar plates.44
Both M. hominis and U. urealyticum may be grown in appropriate artificial broth or agar medium but require somewhat different carbon dioxide concentrations and pH for optimum growth, in addition to the necessary metabolic substrates (i.e., glucose, arginine, urea).45 Mycoplasmas and ureaplasmas are found in a large percentage of the general population. In the obstetric population of Charity Hospital in New Orleans, mycoplasmas were isolated from approximately 50% of gravidas and ureaplasmas from 80%.25 Therefore, proof that these organisms are actually causative of particular disease states is difficult from an epidemiologic and statistical point of view. Proof of pathogenicity is often based on rising antibody liters, although some authors have used culture results, especially blood cultures, to make their point.
Clinical Features
Although M. pneumoniae is the causative agent of human atypical (Eaton-type) pneumonia,43 the association of M. hominis and U. urealyticum with genitourinary and obstetric disease is of interest to the obstetrician-gynecologist.
Ureaplasma has been implicated in NGU14, 46 and the acute urethral syndrome.15 In fact, researchers have produced urethritis in themselves by autoinoculation with strains of U. urealyticum.47 Ureaplasma, like C. trachomatis, may be causative in cases of postgonococcal urethritis, since it is resistant to common gonococcal antibiotic regimens.17
U. urealyticum and M. hominis have been associated with nongonococcal PID, based on direct culture of the organisms from the peritoneum of infected patients.48 Ureaplasma has been isolated from tubo-ovarian abscesses.49 However, since M. hominis is found with equal frequency in infected and noninfected patients,18 and since therapy that is not active against the mycoplasmas is still effective in nongonococcal PID,48 the exact relation of U. urealyticum and M. hominis to the development of PID is unclear.
The genital mycoplasmas have also been implicated in chorioamnionitis, postabortal fever, and postpartum fever. M. hominis has been isolated from women who were febrile after abortion, but patients recovered with or without antibiotics.50, 51 In addition, it has been isolated from the blood of patients with postpartum fever, as well as from those who were afebrile.52, 53, 54 U. urealyticum also has been recovered from patients with chorioamnionitis, but usually other conditions, such as premature or prolonged rupture of the membranes, confound the association.55, 56
Finally, reports link the mycoplasmas to infertility,51, 57 as well as to poor pregnancy outcome.58, 59 Statistics are not conclusive, especially since those patient populations most likely to harbor the genital mycoplasmas are more likely to suffer septic abortion, intrauterine growth retardation, and other abnormal pregnancy outcomes.
Diagnosis
As previously mentioned, both M. hominis and U. urealyticum may be recovered on special media with less difficulty than viral or even anaerobic species.45 Failure to isolate the usual aerobic and anaerobic species from a patient with a syndrome that can be caused by the genital mycoplasmas (e.g., negative urine culture in a patient with dysuria and pyuria) may alert the clinician to the possible presence of these organisms.
Therapy
Since the mycoplasmas have no cell wall, antibiotics that act against bacterial cell walls, notably the β-lactam drugs (penicillins and cephalosporins), are ineffective against these organisms. Tetracycline and its derivatives are effective against most mycoplasma species. M. hominis is sensitive to lincomycin and resistant to erythromycin, whereas U. urealyticum is just the opposite.60
CHANCROID
Chancroid, or “soft chancre,” is an ulcerative STD caused by Hemophilus ducreyi, the streptobacillus of Ducrey. Although one of the original “venereal diseases,” chancroid has become less frequently reported in the United States, possibly because of the increased publicity of the “newer” STDs such as herpes and chlamydia, or because it is a disease more common in low socioeconomic and poor hygienic environments, thus lending itself to higher incidence in third world countries.
Bacteriology
H. ducreyi, like others of the genus Hemophilus, is a small, pleomorphic gram-negative coccobacillus with rather fastidious growth requirements. It may be differentiated from the other Hemophilus species by its requiring factor X (hemin), but not factor V (NAD), for in vitro growth. It may or may not cause hemolysis.61
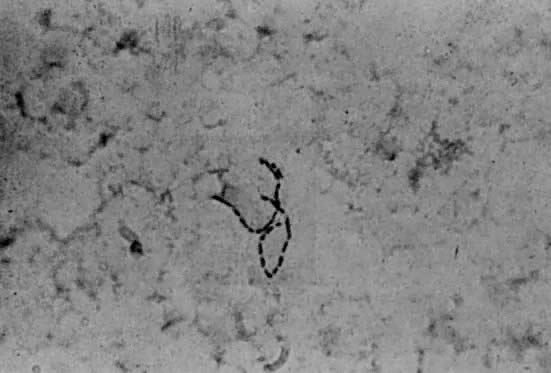
Gram stains of infected areas typically exhibit large numbers of gram-negative coccobacilli in chainlike, or so-called school of fish, pattern (Fig. 3). The organisms may rarely be intracellular. Smears of surface lesions may be difficult to interpret because of contamination; aspirates of buboes are more reliable if positive.62
H. ducreyi may be isolated on chocolate agar treated with isovitalex and vancomycin. Alternately, fresh sterile blood (from the patient) may be inoculated with scrapings from infected areas and subcultured daily to chocolate agar.63
Clinical Features
After an incubation period of 1 to 14 days (usually 2–5), lesions usually arise in the genital area, possibly in areas of small abrasion. In the female, lesions are often on the fourchette, vestibule, labia, clitoris, and anus (very rarely the mouth, fingers, or breasts).
The highly infectious lesion begins as an erythematous papule that becomes pustular and erodes, forming a very painful, sharply demarcated ulcer that is ragged, nonindurated, and surrounded by an erythematous halo. The base of the ulcer bleeds freely and may be covered by necrotic purulent exudate. There is commonly a single lesion, although as many as ten have been reported; they may become confluent, forming a giant, serpiginous ulceration. Superinfection, especially by fusospirochetes, may lead to rapid destruction of the genitalia, the so-called phagedenic chancroid.64, 65
Regional, usually inguinal, adenopathy occurs in one third to one half of patients. Unilateral and painful, the affected nodes may suppurate and rupture spontaneously with fistula formation. Constitutional symptoms, including fever, chills, and malaise, may accompany lymph node involvement.
Diagnosis
Chancroid may be diagnosed clinically by the finding of tender genital ulcer, possibly with unilateral adenopathy. However, genital herpes, superinfected syphilitic chancre, LGV, and granuloma inguinale may present identically; the physician must therefore proceed with more than just a cursory examination and a snap diagnosis.
Smears may be obtained for Gram stain as above. However, even in the presence of a characteristic smear, up to 10% of patients may have simultaneous primary syphilis, so dark-field examination and follow-up serologic studies should be performed. The best material for examination by smear is aspirated from a suppurating lymph node.
Cultures for bacteriologic diagnosis should be attempted. Besides giving definitive identification, isolation of the organism gives the practitioner the availability of sensitivity testing.66
Therapy
The Center for Disease Control recommends treatment of the patient and partner for l0 days with oral erythromycin, 500 mg four times/day, or trimethoprim (160 mg)/sulfamethoxazole (800 mg) twice a day. If adenitis is present, therapy should continue until all nodes are healed. In addition, fluctuant nodes should be aspirated through adjacent normal skin; excision or incision and drainage should be avoided, since this may delay healing.67
Oral sulfisoxazole (1 g four times/day for 1 to 2 weeks) has been a standard therapy. Tetracycline and streptomycin have also been used successfully. However, resistance to sulfonamides and tetracycline has been noted in other countries. Kanamycin (500 mg every 12 hours for 7-14 days) may be used. Cephalothin has also been used.64, 65 Penicillins are not recommended because of documented resistance due to β-lactamase production in some strains.68
While postexposure washing with soaps and disinfectants apparently has no prophylactic effect on the disease, use of condoms may be of benefit. Eradication of sources of continuing carriage (e.g., prostitutes) is a desired public health measure.64
GRANULOMA INGUINALE
Granuloma inguinale, or granuloma venereum, which has been described since 1882, has many synonyms, including granuloma contagiosa, granuloma inguinale tropicum, granuloma pudendi tropicum, sclerosing granuloma, and ulcerating granuloma of the pudenda. Since the causative organism, Calymmatobacterium granulomatis (formerly Donovania granulomatis), was described by Donovan in 1905, the disease has also been termed Donovanosis and granuloma Donovani.
Granuloma inguinale, while one of the five “classic” venereal diseases, is relatively rare in the United States; fewer than 100 cases are reported yearly. However, the disease is very common in subtropical and tropical climates; in some areas up 1:o 25% of the population are infected.69
Bacteriology
C. granulomatis is a pleomorphic gram-negative organism with a prominent capsule and other characteristics that suggest a relationship to the genus Klebsiella. However, C. granulomatis is grown on artificial media only with great difficulty, so physiologic and biochemical parameters of the organisms are thus far incomplete. Therefore the organism is at present classified as somewhat “unaffiliated” from a taxonomic point of view.
Clinical Features
After infection through sexual intercourse or, as in the case of pediatric disease, by close contact with an adult (perhaps through sitting on laps of scantily clad persons), and an ill-defined incubation period of 8 to 80 days, a painless, indurated nodule appears. Singly or in groups, the sore slowly erodes to form an exuberant granulomatous ulcer. The lesion appears heaped and is friable, bleeding easily. Slowly enlarging, multiple sores often coalesce, and new nodules appear through autoinoculation. Secondary infection and postinflammatory fibrosis are not uncommon.64
Hematogenous metastases to bones, joints, and liver have been reported. The lymphatics may be involved, but the apparent bubo formation in inguinal disease is actually a “pseudobubo,” a subcutaneous. granuloma rather than enlarged, suppurative lymph nodes.
In 90% of cases, the genitalia, including the cervix, are the area of primary involvement. Cervical lesions will occasionally resemble cancer. Ten percent of patients will exhibit inguinal involvement primarily, while fewer than 10% will show anal and distant lesions.70
Diagnosis
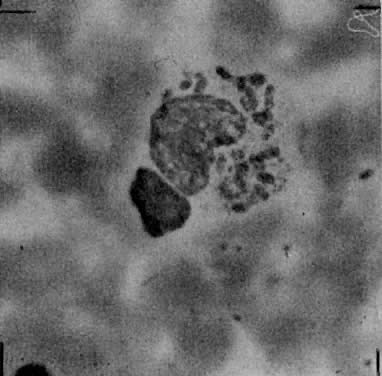
In most cases, clinical examination will suggest the diagnosis. Since culture methods are usually impractical in a clinical setting, a Wright- or Giemsa-stained crush preparation of granulation tissue from a clean lesion will show the characteristic Donovan bodies, clusters of blue-space or black-staining organisms with a “safety pin” appearance in the cytoplasm of large mononuclear cells (Fig. 4). Microscopic examination of formalin-fixed wax-embedded biopsy specimens is less reliable; however, glutaraldehyde-fixed, plastic-embedded electron microscopy specimens readily yield the pathognomonic Donovan bodies.71
Therapy
The first-line antibiotic for C. granulomatis infection is either tetracycline or ampicillin, 500 mg orally every 6 hours; therapy should be continued until lesions heal completely. In many cases ampicillin must be given for 3 months. Lesions should begin to show improvement by the end of the first week of therapy. If the above drugs fail to produce a response or in areas in which resistance is common, chloramphenicol or gentamicin may be the drug of choice.72, 73 Postinfectious scarring and stenosis must be dealt with surgically after the primary infection is controlled.
GROUP B STREPTOCOCCUS
Although isolated in 1935 from the vaginas of puerperal women,74 Streptococcus agalactiae, the group B β-hemolytic streptococcus, was for years considered to be solely an agent of bovine mastitis; streptococci of groups A and D were the only proven human pathogens. However, in 1964 one article reviewed a group of serious infections due to group B streptococci (GBS), and the human pathogenicity of the organisms was stressed, with emphasis on perinatal infections.75
The classification of GBS as an STD is not as clear-cut as with gonorrhea, syphilis, or the other classic venereal diseases. However, the female genital tract has been shown to be frequently colonized, the primary reservoir probably the gastrointestinal tract. Coexistent colonization of the vagina and cervix in the mother and of the urethra in the father have been documented in cases of neonatal disease. The likelihood of sexual transmission is therefore not merely speculative.76
Bacteriology
S. agalactiae is an aerobic, gram-positive coccus, a member of the β-hemolytic streptococci, although up to 5% of isolates do not hemolyze blood. The organism is principally isolated from bovine and human sources, although other animal species occasionally yield strains of GBS.76 The β-hemolytic streptococci were classified serologically by Lancefield, who used the precipitin reaction of group-specific rabbit antisera with cell-wall polysaccharide antigens (so-called C substances).77 A separate envelope carbohydrate (so-called S substance) was then used to separate the group B streptococci into five serotypes: Ia, Ib, Ic, II, and III. Although serotyping is primarily used for epidemiologic purposes, it has been suggested that the type III GBS is a clinically more virulent organism with perhaps some central nervous system (CNS) tropism.78
Although GBS may be differentiated from other streptococci by such techniques as hemolysis reactions, sensitivity to bacitracin (“A disc”), bile-esculin reaction, and hippurate hydrolysis, the CAMP test is the simplest, most reliable method for presumptively identifying GBS short of Lancefield serotyping. The CAMP reaction is based on enhanced lysis of blood agar occurring when GBS is grown in an area of staphylococcal β-hemolysin.79
Clinical Features
GBS has become recognized as the causative .agent of a wide spectrum of human disease; urinary tract infection, bacteremia, gangrene, pneumonia, empyema, meningitis, endocarditis, peritonitis, osteomyelitis, pharyngitis, omphalitis, arthritis, and skin infections have been described.80 However, the syndromes of most interest to the obstetrician-gynecologist are neonatal sepsis and meningitis and maternal puerperal sepsis.
Of the 25% to 35% of pregnant women with vaginal GBS colonization, 26% to 37% will deliver infants colonized by the organism. Of those colonized neonates, approximately 1% will develop the so-called early-onset GBS infection, consisting of severe sepsis, apnea with findings similar to neonatal respiratory distress syndrome, meningitis in 30%, and a case fatality rate of greater than 50%--all this occurring within the first 5 days of life, often the first 48 hours. Risk factors include low birth weight, premature labor, and prolonged rupture of membranes. In the infants with CNS involvement, serotype III GBS is found in over 80%.78
The “late onset” GBS neonatal syndrome consists primarily of meningitis in infants over 5 days of age (mean age 4 weeks). About 20% of these infants succumb, and up to 50% of survivors suffer neurologic sequelae. No apparent risk factors have been identified other than colonization with GBS. The organism may be acquired from a maternal source or from the environment. Over 95% of these isolates are type III.78
Several reports of GBS puerperal infection exist in the literature.75, 81, 82 GBS vaginal colonization has been identified as a risk factor for post-cesarean section morbidity.83 A particular syndrome of severe postpartum endomyoparametritis with onset within 12 hours of delivery, tachycardia, distended abdomen, high fever, and bacteremia in over one third of patients has been attributed to GBS. These patients characteristically exhibit severe uterine and parametrial tenderness. Their lochia is particularly nondescript. The response to appropriate antibiotic therapy is rapid and dramatic, especially if penicillin-type drugs are used (e.g., ticarcillin, mezlocillin, piperacillin).84, 85
Diagnosis
The sine qua non of identification of GBS as the infecting organism in a septic patient is bacterial culture. Isolation of GBS from blood, cerebro-spinal fluid (CSF), urine, or other normally sterile areas is usually no problem. However, GBS is not as hardy as some organisms and may at times be difficult to identify from polymicrobial sources (e.g., cervix, endometrium). A selective medium of Todd-Hewitt broth enriched with sheep blood and treated with gentamicin and nalidixic acid will enhance the isolation of GBS from sites of mixed flora.86
Differentiation of the GBS from other streptococcal strains is done in the manner described previously. The CAMP test is the most easily performed technique; Lancefield grouping is the standard. Serotyping (i.e., types Ia, Ib) is not done as a routine laboratory procedure. Rather, serotypes are important from an epidemiologic standpoint, and serotyping is therefore done only in research laboratories.78
The disease syndromes produced by GBS are often so fulminant, as in early-onset neonatal sepsis, that not uncommonly the clinician must act before culture reports are available. In such cases, Gram stain of clinical material yielding gram-positive streptococci, and indeed, the specific clinical presentation itself (such as in immediate, severe post-cesarean section endomyometritis as described previously) may alert the physician to the possible presence of GBS.
Therapy
The drugs of choice for treatment of GBS infection are ampicillin, penicillin G, and the expanded-spectrum penicillins. GBS is less sensitive to the penicillins than group A streptococci but more sensitive than most enterococci. There may be some inoculum effect at higher concentrations of organism, at least in laboratory testing.87 Disturbingly, there have been some relative failures of the penicillins in GBS infection. There may be tolerant strains arising that are becoming clinically significant in some areas of the United States.chloramphenicol. Vancomycin has also been used. GBS is usually resistant to tetracyclines, and aminoglycosides have almost no activity when used alone. However, as is the case with the enterococci, penicillins and aminoglycosides may be synergistic against GBS, and the combination has been used in vitro and in the mouse model with positive results.87
Although the attack rate in neonatal disease is low, the infections are so fulminant that it is tempting to try prophylaxis of the mother before delivery. 'This is still a matter of intense debate among experts. The prophylactic benefit would be very low, compared with the risk of exposure of a large percentage of the patient population to penicillin and its allergic risk. Confounding circumstances are the carriage of the organism by the male, the fecal reservoir that is difficult to clear with antimicrobials, and the question of timing (i.e., should the gravida be treated at 38 weeks? while in labor? earlier?).76 In addition, GBS infection in the newborn may be related to the inoculum size of the organism on the infant.89 Therefore it would perhaps be advantageous to at least decrease the colony count of the mother, even if she were not completely freed of GBS. Further study in this aspect of GBS microbiology is needed.
HEPATITIS B VIRUS
In 1965, a peculiar antigen was recovered from the blood of an Australian aborigine during investigation of the cause of acute hepatitis.90 The isolation of this material, termed the Australia antigen, was one of the more important epidemiologic discoveries in recent years; this antigen was ultimately found to be a surface antigen of what we now know as hepatitis B virus (HBV).
The case for the labeling of HBV as an STD is strong. Although originally considered primarily spread by parenteral exposure (e.g., needle sticks), up to 50% of infected persons have no such history. Therefore, the term serum hepatitis has become more historic than accurate. The virus is known to be excreted in various body fluids, including feces, semen, and saliva. Studies of spouses of antigen carriers, prostitutes, homosexuals, and patients from STD clinics have implicated sexual contact as an important route of transfer of HBV.91
Bacteriology
HBV is a relatively unique virus in that it contains a double-stranded DNA, and a DNA-polymerase as well, in its intact virion. The virion, presumably the “Dane particle” of early studies, has not been cultured in vitro, leading to difficulty in laboratory study of the virus itself. Only humans and chimpanzees are readily infected with HBV.
Besides the DNA and DNA-polymerase, the virus is composed of surface lipoprotein antigens (HBsAg), which may be grouped into several separate antigenic types for experimental and epidemiologic consideration. Protective antibody elicited by the HBsAg in the human is denoted anti-HBs. Additionally, a core antigen has been identified (HBcAg) that is found in infected hepatocytes. The corresponding antibody (anti-HBc) is found in the serum and is not protective but may be used for diagnostic purposes. Finally, a poorly defined antigen associated with the core of the virus, the “e” antigen (HBeAg), is associated with DNA-polymerase activity (therefore, active viral replication) and may be a marker of infectivity; conversely, the antibody to HBeAg (anti-HBe) may indicate a lessening of infectivity.91
Clinical Features
According to the Centers for Disease Control, the risk of acquiring HBV during one's lifetime is approximately 5% for the US population in general. This figure is higher for patients in some high-risk categories. Approximately 200,000 persons are infected each year; however, only about a fourth of these patients become icteric. HBV accounts directly for some 10,000 hospitalizations :per year and 250 deaths due to fulminant disease. The carrier rate after acute HBV infection is nearly 10%, and chronic active hepatitis (CAH) occurs in about 25% of carriers. In the United States alone, the carrier pool is estimated to be 400,000 to 800,000 persons. CAH often progresses to cirrhosis (leading to about 4000 deaths/year). The HBV carrier state is implicated in the etiology of primary hepatocellular carcinoma (with approximately 800 deaths/year).92
About 90% of HBV infections are self-limited. In the majority of cases, perhaps 75%, the patient develops an asymptomatic HBsAg antigenemia, followed by clearing of the HBsAg and development of anti-HBs. In the remaining patients, an acute icteric hepatitis accompanies the antigenemia; spontaneous resolution occurs as HBsAg disappears and is replaced by anti-HBs.
Approximately 10% of HBV-infected persons will go on to develop a carrier state (normal liver functions, asymptomatic, HBsAg-positive), chronic persistent hepatitis (CPH; abnormal liver functions, asymptomatic, HBsAg-positive), or CAH (abnormal liver functions, symptomatic, HBsAg-positive).
The acute icteric infection caused by HBV is not dissimilar to acute hepatitis due to any other virus, such as hepatitis A virus, Epstein-Barr virus (EBV), or yellow fever. Fever, malaise, liver tenderness, and nonspecific gastrointestinal complaints such as nausea, vomiting, and diarrhea constitute a rather common syndrome when coupled with clinical jaundice, dark-colored urine, and elevated liver transaminases. The majority of patients will clear the virus and heal spontaneously over a period of months. However, a small but significant percentage of HBV-infected patients may develop fulminant liver failure, with elevated blood ammonia levels, coma, and death. In addition, any patient with prolonged (greater than 6 months) elevation of liver enzymes or excretion of HBsAg must be evaluated for CAH, CPH, or the carrier state.
The clinical course of HBV infection in the pregnant patient is said to be no different from that in the nonpregnant woman.93 There is also little evidence for an increased incidence of congenital malformations in these infants, although the rate of spontaneous abortion in HBV-infected women may be increased somewhat over the general population. In addition, nutritional derangement in HBV-infected women may predispose to fetal intrauterine growth retardation.93
The Dane particle is said not to cross the intact placenta. Consequently, maternal HBV infection m early pregnancy does not often lead to direct fetal infection. However, maternal HBsAg antigenemia in the third trimester is an important cause of fetal disease, possibly mediated by microscopic maternal-fetal transplacental hemorrhage, which is increasingly common near term. Contamination of the infant at birth and thereafter is an additional cause of neonatal disease. Mothers who are HBeAg-positive are much more likely to infect their offspring, either in utero or postnatally, than mothers who are HBeAg-negative and who possess anti-HBe.94, 95
Diagnosis
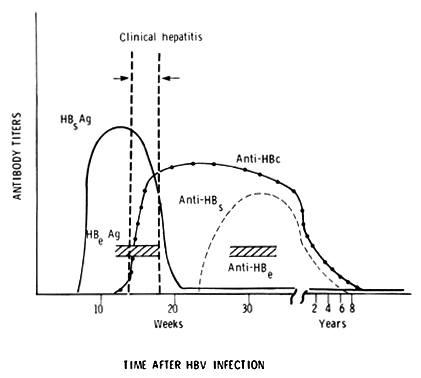
Since HBV is not clinically recoverable, the diagnosis of HBV infection in a patient is made serologically. Serologic tests are available to detect HBsAg, anti-HBs, HBeAg, anti-HBe and anti-HBc (HBcAg is demonstrated only by immunofluorescent studies of liver biopsy specimens). The temporal sequence of the serologic aspects of the disease is depicted in Figure 5.
Upon initial evaluation, a patient with acute HBV infection will probably demonstrate HBsAg antigenemia. However, if examined toward the end of the icteric episode or thereafter, anti-HBs may be demonstrated. The difficulty arises when HBsAg has decreased to undetectable levels and anti-HBs has not yet become detectable; in other words, some patients with acute HBV infection may be HBsAg-negative and anti-HBs-negative. In these cases, anti-HBc may be measured to make the diagnosis. Therefore, to rule out HBV infection in a patient with acute hepatitis, serum should be screened for HBsAg, anti-HBs, and anti-HBc.
Testing for HBeAg and anti-HBe is not particularly helpful in diagnosis. However, presence of HBeAg is possibly a marker of higher infectivity in a patient with HBV infection.
Therapy
There is no known cure for patients infected with HBV. Supportive care and monitoring are usually all that are needed, since the disease spontaneously resolves in most cases. Specific intervention has been attempted in cases of CAH and acute fulminant HBV hepatitis, but only conflicting reports have emerged concerning the use of immune serum and corticosteroids in these diseases.96, 97 There are two available regimens for prophylaxis against HBV infection, however.
Postexposure prophylaxis, for example prophylaxis provided in the case of a contaminated needle stick, has been available for some time. The generally accepted practice has been administration of hepatitis B immune globulin (HBIG), although there are conflicting reports about the efficacy of HBIG when compared with nonspecific immune serum globulin (ISG). In either case, immunity is passively conferred and prophylaxis must be repeated at intervals if continuous or prolonged exposure is anticipated (e.g., dialysis personnel or :spouse of infected person).98
Although preexposure prophylaxis was formerly attempted with either HBIG or ISG, a new inactivated hepatitis B vaccine made from HBsAg obtained by plasmapheresis of carriers was licensed in 1981 in the United States. The vaccine appears to be safe and is highly immunogenic; after the recommended three doses, 95% of persons develop anti-HBs titers, which last for at least 3 years. The vaccine is recommended for immunization of those persons who are HBsAg- and anti-HBs-negative, and who are at risk of HBV infection (e.g., health care personnel, homosexual males, hemodialysis patients, sex partners of carriers).99
A special category of patient is the neonate of the infected (or carrier) woman. All infants born to HBsAg-positive mothers should have cord blood analyzed for HBsAg. If negative, the infant should receive HBIG within 24 to 48 hours of birth, which prevents up to 75% of neonatal infections. The globulin is usually repeated at 3 and 6 months. However, with the advent of HBV vaccine, the infant may be vaccinated at 3 months of age if the mother is a chronic carrier. Studies are still underway to determine the optimal combination of HBIG and HBV vaccine yielding the best results.92
CYTOMEGALOVIRUS
In 1956, three separate laboratories working on three different microbiologic problems isolated the viral agent that causes cytomegalic inclusion disease (CID) in fetuses and neonates, Previously, CID was presumed to be caused by protozoans, although the characteristic intranuclear inclusions had been known for years.100 It is now believed that the cytomegalovirus (CMV) is the leading cause of intrauterine infection in man.101 Circumstantial evidence incriminates CMV as a venereally transmitted disease.
Bacteriology
CMV is a DNA virus of the herpesvirus group, along with varicella-zoster, EBV, and the two herpes simplex viruses. These viruses share the properties of cell association, the ability to propagate best in infected cells, and latency, the ability to remain clinically inactive in the host and then to reactivate. Along with the other herpesviruses, CMV may be implicated in oncogenesis. CMV can, under special conditions, transform hamster cells into tumor. Its effects on cells also resemble those of other oncogenic viruses, such as EBV and Simian SV-40.102
CMV is phylogenetically a very old virus. The strains of CMV have become so specialized that they are extremely species specific; although human, simian, murine, equine, bovine, and even hedgehog strains of CMV have been isolated, the individual strains will grow only in their appropriate host tissues. Consequently, animal models of human CMV infection are nonexistent, and cell culture isolation can be accomplished only using human cell lines. Usually cell cultures of human foreskin or embryonic skin or muscle are used.103
Human CMV exists as multiple serotypes, causing confusion in clinical and epidemiologic study. The viral strain most frequently used in serologic testing is the ADI69 strain; this strain has enough common antigens with most other serotypes to be widely usable in serologic methods. Controversy still ensues as to whether complement fixation (CF) tests using AD169 are sensitive enough in all situations to be used diagnostically. Indirect fluorescent antibody (IFA) and anticomplement immunofluorescent tests (ACIF) are more sensitive but more difficult to perform.104, 105
Whatever the method of measurement, serologic evidence of CMV infection is extremely high, from 40% to 100% of persons tested, depending upon the population; higher rates are found in “less developed ”countries in Africa and Southeast Asia. Antibodies may be found in about 50% of infants at birth, but this is largely transplacentally acquired IgG antibody. By one year of age, fewer than 10% of infants have positive antibody titers for CMV. Seropositivity increases with advancing age; teenagers have an approximate 25% rate of seropositivity, adults over 50% (in the US).102
The source of CMV in cases of infection is poorly understood. The virus is ubiquitous and excreted in milk, saliva, semen, feces, blood, urine, and cervicovaginal secretions. It is estimated that up to 2% of infants are infected in utero, usually due to maternal viremia. Up to 30% of children then become infected by vaginal secretions at delivery, breast milk, or respiratory and oral droplets.106
Additional cases of new CMV infection may be associated with blood transfusion.107 Also. CMV runs in the pack of other STDs and is more common in patients in STD clinics. Viral titers are unusually high and persistent in semen. Cervical shedding is also important and seems to rise in the latter stages of pregnancy. An additional source of CMV infection is the human tissue allograft (e.g., kidney transplant). Infections acquired through this route may be unusually troublesome owing to the accompanying immunosuppression.102
Clinical Features
Of the roughly 2% of infants infected with CMV in utero, the vast majority will be born without visible abnormalities. It is generally accepted that infants infected earlier in pregnancy are at more risk for congenital abnormalities in pregnancies in which the mother suffers CMV viremia. If the child does show evidence of CMV infection at birth, the prognosis is very poor for normal mental capacity. Even among patients completely asymptomatic at birth, approximately 10% will develop learning disabilities, deafness, microcephaly, and school failure. In addition, behavior difficulties seem to be greater in CMV-infected infants followed for 3 to 5 years.108 CMV-induced perinatal diseases are listed below:
First Trimester
Microcephaly
Encephalitis
Cerebral calcifications
Seizure disorder
Perceptual handicaps (e.g., deafness)
Mental retardation
Hepatosplenomegaly
Hepatitis
Pericarditis
Myocarditis
Pneumonia
Anemia
Coagulopathy
Thrombocytopenia
Second trimester
Gradations of first trimester effects
Third trimester
Asymptomatic
Mild retardation
Perceptual handicaps
Deafness
Microcephaly
Learning disability
Chronic CMV Shedding
Adult disease is largely asymptomatic. A few persons will manifest a heterophile-negative mononucleosis-type syndrome with fever, malaise, splenomegaly, possible hepatitis, and peripheral blood atypical lymphocytosis. This syndrome differs somewhat from true mononucleosis due to EBV infection by the rather minor lymphadenopathy and tonsillar hypertrophy of CMV mononucleosis in contrast to the marked adenopathy found with EBV mononucleosis. The lymphocytosis is also not as pronounced in CMV disease. This syndrome is possible with any type of CMV infection, but it is more common if the infection is preceded by blood transfusion or if the patient is immunosuppressed. In the immunosuppressed patient, a wider variety of problems may manifest, including pneumonitis, retinitis, and encephalitis.100
Diagnosis
Diagnosis of CMV infection is fraught with pitfalls. A positive culture may mean nothing, since viral shedding is most often asymptomatic and may merely be coincidental to the disease being investigated. Similar comments may be made concerning the finding of typical enlarged cells with CMV inclusions in histologic preparations of urine, semen:, or other fluids. Serologic methods are also difficult to interpret because a rise in antibody titer may not accompany clinical disease. In addition, the virus causing the disease may not be sufficiently similar to the test strain used in the serology laboratory.
In infants, the situation is a bit easier. Cord blood with anti-CMV IgM (measured by immunofluorescent test) is over 75% accurate in diagnosing intrauterine infection, while the detection of CMV-specific nuclear antigens by ACIF in specimens from collected urine is over 90% accurate. In addition, electron microscopy of urine sediment may provide a diagnosis within an hour.109
Therapy
Antiviral drugs have been used in small numbers of infected infants as well as in adults. While vital excretion was decreased during therapy, clinical disease was unchanged, and virus reappeared after discontinuation of the drugs.100
Vaccine derived from the Towne-125 strain of CMV has proven immunogenic and relatively safe; both humoral and cellular immunity have been provoked.110, 111 The problem with the concept of vaccination is that in natural disease, serologic immunity does not necessarily correspond to clinical immunity or protection of the fetus from infection. In addition, infecting a patient purposefully with a live DNA virus with the potential for latency or oncogenicity must be done only after great deliberation.
LISTERIA MONOCYTOGENES
The causative organism of listeriosis has had an interesting history, paralleled by its multiple former names. Bacterium monocytogenes was noted to cause sepsis and peripheral monocytosis in laboratory animals in 1926. Listerella hepatolytica was noted to cause hepatitis in gerbils in 1927. In 1929, a case of human infectious mononucleosis was accompanied by bacteremia with a similar organism. Other names probably describing L. monocytogenes have been Corynebacterium infantisepticum, Corynebacterium parvulum, and Erysipelothrix monocytogenes. Perinatal disease and meningitis in humans were described in the mid 1930s, and further reports of the disease occurring during pregnancy or in the perinatal period have appeared in the obstetric: and the pediatric literature subsequently.112
Bacteriology
L. monocytogenes is a gram-positive, facultative anaerobic rod that is morphologically similar to the diphtheroid group. However, L. monocytogenes is hemolytic on blood agar. In clinical Gram stains, Listeria may be somewhat coccoid and occur in pairs; it may therefore be mistaken for gram-positive streptococci (e.g., GBS, pneumococcus). Also, overdecolorization of the Gram stain may cause the organisms to resemble some gram-negative species, especially the coccobacillary Hemophilus influenzae. It is therefore incumbent upon the laboratory to be certain of technique and not overlook diphtheroids as contaminants without at least some caution.112
There are eleven serotypically separable strains of L. monocytogenes. However, over 90% of human disease is caused by strains Ia, Ib, and IVb, a factor of epidemiologic interest but of little clinical importance.
The epidemiology of L. monocytogenes is difficult to complete. The organism is widespread in nature, including soil, water, and sewerage, and is found in nearly every type of animal from fish to flies.113, 114 Listeriosis has been regarded as a zoonosis associated with milk and raw beef; however, fecal excretion seems to have some relation to human carriage.115
Clinical Features
Listeriosis is a disease of neonates, the aged, and pregnant women; the organism rarely attacks a. completely immunocompetent host. From one third to two thirds of cases occur during pregnancy and the neonatal period.115 The disease caused by L. monocytogenes may be divided for obstetric purposes into maternal disease and neonatal disease.
Maternal infection by L. monocytogenes is usually asymptomatic, consisting merely of genital carriage. There may be some aspect of venereal transmission, since males generally harbor the organism concomitantly. If symptomatic, maternal illness consists of a flulike syndrome, with fever, malaise, coryza, and abdominal pain. Bacterial endocarditis due to L. monocytogenes during pregnancy has been reported in a diabetic patient.116 It is usually possible to document bacteremia in cases of symptomatic maternal disease, although the disease usually subsides with or without therapy.
Transplacental transmission of Listeria may cause a unique syndrome, granulomatosis infantiseptica, in which the infant suffers disseminated abscesses and granulomas of multiple internal organs, including the placenta. This so-called early disease is fulminant and often fatal; therefore prompt therapy is imperative even if the diagnosis is only tentative.112
The second form of pediatric disease is the “late disease” consisting of sepsis, often with meningoencephalitis, after 3 or more days of life. The source of the bacteria is probably vaginal contamination. Occasionally a peripheral monocytosis may suggest the etiology although the clinical presentation is fairly nonspecific.12
Diagnosis
L. monocytogenes may be cultured by the usual methods with the cautions mentioned above. The most important consideration is a high index of suspicion and clinical awareness. Diphtheroid organisms in appropriate clinical situations should be thoroughly investigated.
Therapy
Listeriosis has been the subject of few controlled studies. A 2-week course of ampicillin or penicillin appears to be the therapy of choice, although some degree of penicillin resistance has been reported. Other possible treatment regimens include tetracyclines, erythromycin, cephalosporins, and chloramphenicol. It must be emphasized that antibiotic failures in severe disease may be due to late institution of therapy. By the same token, adult disease is often mild and self-limiting, so that bacterial culture is the only way to document cure.112
One review suggests that antepartum antibiotic therapy without delivery in cases of maternal septicemia may be an important concept. The risk of respiratory distress syndrome in the neonate due to premature delivery is avoided by delaying delivery. The study is retrospective, however, and the subject needs further investigation.117
THE ENTERITIDES
Although not usual inhabitants of the female genital tract, several organisms that classically cause gastroenteritis and, in many cases, systemic illness have been reported to invade the internal and external female genitalia. While this may be the result of systemic dissemination, anal intercourse must not be overlooked as a possible source of these enteric pathogens. The two organisms considered here are the protozoan Entamoeba histolytica and the pathogenic bacteria of the Shigella group.
Bacteriology
E. histolytica belongs to subphylum Sarcodina, a group of :protozoans with pseudopod-mediated motility. E. histolytica is antigenically distinct from other entamoebas and is also rather unique in its pathogenic potential for humans. E. histolytica exists in two stages: the trophozoite, which is the active, motile form of the organism; and the cyst, which is inactive, but which is the usual agent of transmission.
The E. histolytica trophozoites are facultatively anaerobic and survive well in the environment provided by the colon. Conditions that allow invasion of tissues are not fully understood. However, minute trauma to mucosal surfaces can facilitate invasive disease. The trophozoites, which reproduce by binary fission, are rapidly destroyed by gastric acidity and thus are not the source of spread of the disease.
The E. histolytica cyst is usually formed in the colon, presumably during times when conditions are less than optimum for the growth of the trophozoites. Maturation takes place either in the bowel lumen or in an external moist environment. When the cyst is ingested, it dissolves in the small bowel and releases eight trophozoites. Thus, the cyst is the medium of infectivity, usually residing on or in contaminated vegetable matter or drinking water. Infections with E. histolytica, therefore, are more common in areas of lower socioeconomic status (i.e., developing countries), although outbreaks in technologically advanced countries are associated with transiently contaminated water supplies.118
Epidemiologically similar is the Shigella species, the etiologic agents of bacillary dysentery. These are gram-negative aerobic rods, members of the family Enterobacteriaceae. As with many gram-negative organisms, serotyping separates the Shigella into some 40 serotypes, which in turn may be arranged into four major groups based on serologic similarities and carbohydrate fermentation tendencies. These are Shigella dysenteriae (group A), S. flexneri (group B), S. boydii (group C), and S. sonnei (group D). S. sonnei accounts for the majority of cases in the United States. The fly may serve as a vector for the organism; hence, disease outbreaks often occur during the summer.119
Clinical Features
E. histolytica attacks primarily the gastrointestinal mucosa, causing an ulcerative disease. The usual site of involvement is the colon, with diarrhea being the most common complaint. However, the organism may disseminate by invasion of the submucosal vasculature. The most common metastatic site is the liver, owing to the enterohepatic flow of blood. Rare spread to other distant organs such as brain and skin is encountered. More commonly the perineal area is infected by direct contact with infected feces. In addition, diseased bowel or liver may perforate intraperitoneally, causing an inflammatory and occasionally granulomatous peritonitis.118
In females with intestinal amebiasis, the most frequent genital lesion is an ulcerative disease of the vulva and vagina.120, 121 Similar ulcerations, including friable exophytic granulations that suggest carcinoma, may be seen on the cervix. Urethral inflammation may accompany vaginitis by E. histolytica.
Perforation of affected bowel may, owing to gravity drainage, allow the formation of pelvic, and especially cul-de-sac, abscesses due to ameba. These may become secondarily infected and clinically difficult to distinguish from pelvic or tubo-ovarian abscesses of the more usual etiology. Distal bowel perforation may lead to amebic genital fistulas, such as rectovaginal and rectouterine fistulas in the female. The cause of these lesions must be ascertained, since surgical correction is contraindicated before appropriate medical therapy.122
Bacillary dysentery is the common syndrome associated with Shigella infections. The patient may have severe diarrhea, possibly with significant bloody or mucoid component. Systemic toxicity, with high fever, chills, abdominal pain, and hyperactive bowel sounds, is the hallmark of the full-blown syndrome. In compromised patients (i.e., infants and the elderly), dehydration may be rapid. However, the disease is usually self-limiting, resolving in from a few days to a month in the otherwise healthy person.119
In the female, S. sonnei has been implicated in salpingitis and the Fitz-Hugh-Curtis syndrome. A patient who practiced anal intercourse and had a history of an acute diarrheal illness was found to harbor S. sonnei in great numbers on the cervix, associated with symptoms of PID and perihepatitis.123
Diagnosis
Gastrointestinal amebiasis may be diagnosed by :finding the characteristic cysts or trophozoites of E. histolytica in stool specimens. If the number of organisms is low, the ameba may be cultured on .egg enrichment medium from fresh stool. Serology is usually negative in uncomplicated cases; positive titers may be indicative of invasion.118
In cases of female genital amebiasis, the cysts and trophozoites may be identified in the Papanicolaou smear. The general pattern of the smear is that of inflammatory reaction, cellular debris, and histiocytes. The ameba must be differentiated from trichomonads and large mononuclear cells.124
Shigella species are readily cultured on MacConkey or other media specific for the aerobic enteric organisms. They may also be grown on Salmonella-Shigella media. The difficulty arises in knowing when to culture for the organism specifically from places such as the cervix or vagina.
Therapy
Prevention of amebiasis rests with hygienic sewerage disposal methods. Prompt treatment of gastrointestinal infection and good perineal and vaginal hygiene will usually prevent gynecologic disease. The organism itself is treated with various regimens, depending upon the situation. Asymptomatic gastrointestinal carriage may be treated with tetracycline or doxycycline for a week, combined with diiodohydroxyquin for 3 weeks. Symptomatic amebiasis should be treated with metronidazole for 5 days if limited to the gastrointestinal tract and for up to 10 days if extraintestinal infection exists. Both of these regimes are accompanied by diiodohydroxyquin to prevent the carrier state.118
Shigellosis may be treated with tetracycline or ampicillin. However, sensitivity studies should be performed, since antibiotic resistance is not uncommon. Resistant strains may respond to trimethoprim/sulfamethoxazole combinations. Treatment regimens should be continued for 5 days to a week and the patient recultured for confirmation of cure.119
SCHISTOSOMA SPECIES
The human blood flukes Schistosoma mansoni, S. japonicum, and S. haematobium cause a large proportion of human flatworm infections. More than 200 million persons are infected worldwide and the incidence is on the increase. Although gynecologic infections are a small part of the whole, they are still significant, especially in countries in which the disease is endemic.125
Bacteriology
The schistosomes are flatworms of the class Trematoda. The human pathogens are parasites that reproduce sexually in the adult form and asexually in the larval stages. The human is the definitive host, the adult worms living in the venous system of the intestines (S. japonicum, superior mesenteric veins; S. mansoni, inferior mesenteric veins) or the bladder (S. haematobium, vesical venous plexus). Eggs are passed through stool or urine and hatch in fresh water, where a larval stage (the miracidia) infects a specific species of snail as an intermediary host. The miracidia multiply asexually in the body of the snail and emerge in 4 to 6 weeks as hundreds of motile cercariae, which are infective. The cercariae may penetrate intact skin, becoming schistosomula, and migrate to lungs and liver. Maturation takes about 6 weeks, and the adult worms then migrate through the venous system to their final habitat.
The snail intermediate host is species specific. Therefore, the various Schistosoma species may not complete their life cycle in areas where their specific snail species is absent. Fortunately the United States is devoid of these particular species. Endemic areas include Puerto Rico, the Philippines, Brazil, and the Middle East. Therefore, travelers to these areas, as well as immigrants from these countries, are at risk.126
Clinical Features
Within 24 hours of penetration of the skin by cercariae, a papular skin rash known as swimmer's itch may appear. The next symptoms appear in 1 to 2 months, at the time of egg laying; a syndrome of acute fever, chills, sweating, headache, and cough (called Katayama fever in Japan) accompanied by peripheral eosinophilia occurs, more commonly in infections with S. japonicum or heavy infestations with S. mansoni. Death may ensue in severe cases, but recovery usually occurs in several weeks. Patients with mild to moderate “worm loads” are thereafter asymptomatic.126
Female genital infection usually presents with a foul vaginal discharge, possibly irregular bleeding, and varying forms of infertility and chronic abortion. Examination may reveal cervical ulcers, erosions, or polyps; there may be adnexal thickening and forniceal fullness; uterine hypertrophy may be apparent. Hyperplastic, warty vulvar and polypoid or ulcerated vaginal lesions are not uncommon.127
Schistosomes have been demonstrated to invade the placenta and fetus. Other intraperitoneal infections, such as a PID-like tubal infection, have been reported.128 Female genital schistosomiasis has been coincidentally found with gynecologic cancers. However, no cause-and-effect relationship seems to exist.
Diagnosis
Since schistosomiasis is a geographically restricted disease, inquiry about travel to endemic areas may serve to rule out the disease. History of swimmer's itch followed appropriately by the acute febrile syndrome is highly suspicious. Definitive diagnosis is made only by demonstrating eggs in feces, rectal biopsy, or urine.
Although cytologic specimens (e.g., Papanicolaou smears) of the female genital tract may reveal forms diagnostic of schistosomiasis, this method should not be used exclusively for diagnosis, since it has a high degree of insensitivity (many false negatives) when compared with standard methods.130
Therapy
Since schistosomiasis is an essentially self-limiting disease and rather harmless in cases of low worm count, patients are usually not treated. Management is generally aimed at prevention. In severe cases, it may be of benefit to reduce the worm count with niridazole or stibocaptate, two relatively toxic drugs available from the Centers for Disease Control in Atlanta.126
SYSTEMIC MYCOSES
Two of the more serious fungal infections in the United States are North American blastomycosis or Gilchrist's disease, and coccidioidomycosis, often called San Joaquin Valley fever. Both of these entities usually manifest as systemic disease; however, gynecologic lesions, especially of the vulvar skin, may occur.
Bacteriology
North American blastomycosis is caused by the dimorphic fungus Blastomyces (Ajellomyces) dermatitidis. The organisms exist in the mycelial phase at room temperature and in a yeastlike phase at 37°C (98.6°F) (both in vitro and in vivo). The yeastlike form is a single cell with one bud connected to the parent cell by a wide neck. The organism is endemic to the basins of the Mississippi, Ohio, and St. Lawrence rivers.131
Coccidioidomycosis is caused by the saprophytic soil fungus Coccidiodes immitis, which is endemic to the Southwestern United States and parts of Central and South America. The fungus exists in the soil in the mycelial phase, forming arthroconidia as it matures. The arthroconidia act as spores and become airborne, infecting soil or animals (by inhalation). In an animal host, a thick-walled spherule develops that reproduces by formation of up to 800 endospores. The spherule may rupture, releasing endospores that develop into new spherules if retained in the body or revert to the mycelial phase if excreted into the soil.132
Clinical Features
Both B. dermatitidis and C. immitis may be contracted by inhalation or by cutaneous abrasion. The primary disease caused by both pathogens is pulmonary, consisting of a febrile illness with cough, chest pain, and roentgenographic abnormalities. Coccidioidomycosis is often self-limiting, while blastomycosis is progressive and fatal in a majority of untreated patients. Hematogenous dissemination may occur with both fungi; in untreated pregnant women C. immitis is much more likely to disseminate and invade distant organs. 131, 132
Cutaneous manifestations of blastomycosis occur in up to 80% of patients and. include subcutaneous nodules or papulopustules that ulcerate. The borders advance and the center heals with scarring. Isolated mucous membrane lesions are not common. The most common gynecologic presentation is therefore vulvar cutaneous blastomycosis. Similar disease is produced by C. immitis, the most characteristic finding is a warty proliferative lesion that may encompass wide areas of skin if untreated.
Diagnosis
Both B. dermatitidis and C. immitis may be grown on standard media such as Sabouraud's. Presumptive diagnosis may be made by demonstrating the blastomycotic yeast form or the coccidioidomycotic spherule in wet preparations of exudates or fluids and in biopsy specimens.
Serum complement-fixing antibodies are not particularly useful in blastomycosis, since only 50% to 70% of cases are positive. Similarly, only .40% to 50% of patients with disseminated coccidioidomycosis have elevated titers, although there is a specific skin test with coccidioidal antigen that becomes positive from 2 to 21 days after first symptoms appear.
Therapy
All patients with blastomycosis should be treated, since the disease is potentially fatal. Amphotericin B is the drug of choice, with 1-hydroxystilbamidine a second-line drug. The toxicity of these drugs demands a firm diagnosis before therapy is begun.131
Coccidioidomycosis is often a self-limiting illness and therefore may go without treatment. Patients with severe primary disease, as well as pregnant patients and other immunocompromised persons, should receive amphotericin B. In addition, patients with high complement-fixing antibody titers and those with chronic pulmonary or osseous disease should be treated.
ACTINOMYCOSIS
Actinomycosis is a chronic, indolent, granulomatous disease caused by the oral commensal species of Actinomyces and Arachnia. Human infections are most commonly caused by Actinomyces israelii. Gynecologic infections are usually associated with use of intrauterine contraceptive devices (IUDs).
Bacteriology
A. israelii is an anaerobic, gram-positive, filamentous bacteria that was formerly considered a fungus. The organism is also partially acid fast. On agar media the bacteria is slow growing, producing delicately branching “spider” colonies initially, maturing in a week into large, white, opaque, “molar tooth” colonies. In infected tissues, colonies characteristically form the so-called sulfur granules, which are distinct conglomerate masses of microorganisms mixed with inflammatory debris.133
Clinical Features
Actinomycosis classically presents as either orofacial disease, thoracic disease, or abdominopelvic disease. The former two entities result from invasion of endogenous oral A. israelii into the appropriate tissues by dental infection or by aspiration or esophageal penetration. The resulting infection is a spreading, pyogenic lesion, not confined to tissue planes, which may erupt through overlying skin, forming draining sinuses. Pulmonary disease may resemble carcinoma or tuberculosis.
Abdominal disease usually follows surgery for inflammatory bowel disease, appendicitis, penetrating trauma, and the like. The disease may mimic carcinoma, amebiasis, or tuberculosis and is characterized by microabscess, chronic sinus and fistula formation, and “woody” phlegmon.134
Colonization of the cervix with A. israelii occurs in up to 5% of IUD users, although nonexistent in nonusers.135 Most cases are asymptomatic. However, women with actinomycetes identified on Papanicolaou smear of the cervix have been shown to have a 3.6-fold greater incidence of hospitalization for PID. Colonized women with PID also had a higher rate of tubo-ovarian abscess compared with noncolonized women and were more often treated surgically.136
Fatalities have been reported, as represented by the following case reported from Charity Hospital in New Orleans:137
A 23-year old woman was admitted with fever, chills, and lower abdominal pain and swelling. The patient had been treated for PID several months earlier during which time a pelvic abscess was drained, forming a draining sinus.
Upon examination, she had a mass filling the pelvis and extending into the lower abdomen. The sinus tract appeared to be connected to this mass. Diagnostic tests for tuberculosis and fungus were negative.
The patient's hospital course spanned 7 months of improvement and relapse. The patient died in spite of blood transfusion and intensive supportive care.
At autopsy, a thick-walled conglomerate mass filled the pelvis and lower abdomen. The impression at that time was that the initial disease had originated in the tubes and ovaries.
Microscopic sections of the mass (Fig. 6) revealed the remains of the left tube with foci of typical Actinomyces colonies, surrounded with inflammatory cells and debris.
|
Diagnosis
A. israelii may be cultured anaerobically, although identification may take up to a week or more. Female genital colonization may be detected by Papanicolaou smear or by direct examination of the removed IUD. There is some evidence that serologic testing may prove useful in diagnosing genital actinomycosis.138
Therapy
Prolonged antibiotic therapy and surgical drainage are the.. proven management for actinomycosis. Penicillin in high doses is the drug of choice and may be sufficient without surgical intervention. Parenteral therapy for 4 to 6 weeks and oral therapy for 6 to 12 months is recommended. Chloramphenicol, erythromycin, and tetracyclines have also been used successfully.133
In the case of actinomycotic genital colonization in an IUD wearer, treatment is still controversial. Conservative management would be removal of the IUD and simple follow-up with repeat Papanicolaou smear after the next menstrual period.135 Some feel, however, that colonized women should be treated with appropriate antibiotics.139
REFERENCES
Gordon FB, Quan AL: Isolation of the trachoma agent in cell culture. Proc Soc Exp Biol Med 118: 354, 1965 |
|
Bowie W, Holmes KK: Chlamydial diseases. In Mandell GL, Douglas RG, Bennett JE (eds): Principles and Practice of Infectious Diseases, vol. 2, p 1461. New York, John Wiley & Sons, 1979 |
|
Grayston JT, Wang SP: New knowledge of Chlamydiae and the disease they cause. J Infect Dis 132: 87, 1975 |
|
Schacter J, Dawson CR: Comparative efficiency of various diagnostic methods for chlamydial infection. In Hobson D, Holmes KK (eds): Nongonococcal and Related Infections, pp. 337–341. Washington, DC, American Society for Microbiology, 1977 |
|
Schacter J: Chlamydial infections. N Engl J Med 298: 540, 1978 |
|
Gupta PK, Lee EF, Erozan YS et al: Cytologic investigations in Chlamydia infections. Acta Cytol 23, No. 4: 315, 1979 |
|
Purola E, Paavonen J: Cytologic findings in cervical chlamydial infection. Med Biol 58: 174, 1980 |
|
Schacter J: Can chlamydial infection cause rheumatic disease? In Damonde DC (ed): Infection and Immunology in the Rheumatic Diseases, p 151. Oxford, Blackwell Scientific, 1976 |
|
Grayston JT, Mordhost CH, Wang S: Childhood myocarditis associated with Chlamydia trachomatis infection. JAMA 246, No. 24: 2823, 1981 |
|
Bel-Kahn JM, Watanakumakarn C, Menefee MG et al: Chlamydia trachomatis endocarditis. Am Heart J 95: 627, 1978 |
|
Thygeson P: Historical review of oculogenital disease. Am J Ophthalmol 71: 975, 1971 |
|
Beem MO, Sazon EM: Respiratory tract colonization and a distinct pneumonia syndrome in infants infected with Chlamydia trachomatis. N Engl J Med 139: 232, 1979 |
|
Dunlop EMC, Harper IA, AI-Hussaini MK et al: Relation of TRIC agent to “nonspecific” genital infection. Br J Vener Dis 42: 77, 1966 |
|
Holmes KK, Wang SP, Handsfield HH et al: Etiology of nongonococcal urethritis. N Engl J Med 292: 1199, 1975 |
|
Stamm WE, Wagner KF, Amsel R et al: Causes of the acute urethral syndrome in women. N Engl J Med 303: 409, 1980 |
|
Weil A, Gaudenz R, Burgener L et al: Isolation of Chlamydia trachomatis from women with urethral syndrome. Arch Gynecol 230: 329, 1981 |
|
Holmes KK, Johnson DW, Floyd TM et al: Studies of venereal disease: II. Observations on the incidence, etiology and treatment of the postgonococcal urethritis syndrome. JAMA 202: 467, 1967 |
|
Mardh PA, Ripa T, Svensson L et al: Chlamydia trachomatis infection in patients with acute salpingitis. N Engl J Med 296: 1377, 1977 |
|
Paavonen J, Saikku P, Vesterinen E et al: Chlamydia trachomatis in acute salpingitis. Br J Vener Dis 55: 203, 1979 |
|
Ripa KT, Svensson L, Treharne JD et al: Chlamydia trachomatis infection in patients with laparoscopically verified acute salpingitis. Am J Obstet Gynecol 138: 960, 1980 |
|
Gjonnaess H, Dalaker K, Anestad G et al: Pelvic inflammatory disease: Etiologic studies with emphasis on chlamydial infection. Obstet Gynecol 59: 550, 1982 |
|
Delaker K, Gjonnaess H, Kuile G et al: Chlamydia trachomatis as a cause of acute perihepatitis associated with pelvic inflammatory disease. Br J Vener Dis 57: 41, 1981 |
|
Henry-Suchet J, Catalan F, Loffredo V et al: Chlamydia trachomatis associated with chronic inflammation in abdominal specimens from women selected for tuboplasty. Fertil Steril 36: 599, 1981 |
|
Persson K, Ronnerstam R, Svanberg L et al: Maternal and infantile infection with Chlamydia in a Swedish population. Acta Paediatr Scand 70: 101, 1981 |
|
Martin DH, Faro S, Pastorek JG: High prevalence of chlamydial infections in an inner city obstetrical population. Twenty-first ICAAC, Chicago, 1981 (abstr 515) |
|
Martin DH, Koutsky L, Eschenbach DA et al: Prematurity and perinatal mortality in pregnancies complicated by maternal Chlamydia trachomatis infection. JAMA 247: 1585, 1982 |
|
Cytryn A, Purnendu S, Haingsub R et al: Severe pelvic infection from Chlamydia trachomatis after cesarean section. JAMA 247: 1732, 1982 |
|
Wagwer GP, Martin DH, Koutski L et al: Puerperal infectious morbidity: Relationship to route of delivery and to antepartum Chlamydia trachomatis infection. Am J Obstet Gynecol 138: 1008, 1980 |
|
Bowie W, Holmes KK: Chlamydia trachomatis. In Mandell GL, Douglas RG, Bennett JE (eds): Principles and Practice of Infectious Diseases, pp. 1469–1470. New York, John Wiley & Sons, 1979 |
|
Wentworth BB, Alexander ER: Isolation of Chlamydia trachomatis by use of 5-iodo-2-deoxyuridine-treated cells. Appl Microbiol 27: 912, 1974 |
|
Hare MJ, Toone E, Taylor-Robinson D et al: Follicular cervicitis-colposcopic appearances and association with Chlamydia trachomatis. Br J Obstet Gynecol 88: 174, 1981 |
|
Paavonen J, Vesterinen E, Meyer B et al: Colposcopic and histologic findings in cervical chlamydial infection. Obstet Gynecol 59: 712, 1982 |
|
Bowie WR, Lee CK, Alexander ER: Prediction of efficacy of antimicrobial agents in treatment of infections due to Chlamydia trachomatis. J Infect Dis 138: 655, 1982 |
|
Bowie WR: Lack of in vitro activity of cefoxitin, cefamandole, cefuroxime and piperacillin against Chlamydia trachomatis. Antimicrob Agents Chemother 21: 339, 1982 |
|
Centers for Disease Control: Sexually transmitted diseases treatment guidelines 1982. MMWR (Suppl): 31:355, 1982 |
|
Bowie WR, Alexander ER, Floyd JF et al: Differential response of chlamydial and ureaplasma-associated urethritis to sulfurazole (sulfisoxazole) and aminocyclitols. Lancet 2: 1276, 1976 |
|
Johannisson G, Sernryd A, Lyke E: Susceptibility of Chlamydia trachomatis to antibiotics in vitro and in vivo, J Am Vener Dis Assoc 6:50, 1979 |
|
American Academy of Pediatrics: Prophylaxis and treatment of neonatal gonococcal infections. Pediatrics 65:1047, 1980 |
|
National Society to Prevent Blindness: Prevention and treatment of ophthalmia neonatorum. New York, National Society to Prevent Blindness, 1981 |
|
Hammerschlag MR, Chandler JW, Alexander ER et al: Erythromycin ointment for ocular prophylaxis of neonatal chlamydial infection, JAMA 244:2291, 1980 |
|
Nocard E, Roux ER: Le microbe de la peripneumoniae. Ann Inst Pasteur 12: 240, 1898 |
|
Dienes L, Edsall G: Observations on l-organisms of Klieneberger. Proc Soc Exp Biol Med 36: 740, 1947 |
|
Chanock RM, Hayflick L, Barlie MF: Growth on artificial medium of an agent associated with atypical pneumonia and its identification as PPLO. Proc Natl Acad Sci USA 48: 41, 1962 |
|
Couch RB: Mycoplasma diseases. In Mandell GL, Douglas RG, Bennett JE (eds): Principles and Practice of Infectious Diseases, p 1481. New York, John Wiley & Sons, 1979 |
|
Kenny GE: Mycoplasmata. In Lenette EH, Balows A, Hausler WJ et al (eds): Manual of Clinical Microbiology, 3rd ed, p. 365. Washington, DC, American Society of Microbiology, 1980 |
|
Shepard MC: Non-gonococcal urethritis: Association with the human strain of “T” mycoplasma. JAMA 211: 1335, 1970 |
|
Taylor-Robinson D, Csonka GW, Prentice MJ: Human intraurethral inoculation of ureaplasmas. QJ Med 46: 309, 1977 |
|
Thompson SE, Hager WD, Wong KH et al: The microbiology and therapy of acute pelvic inflammatory disease in hospitalized patients. Am J Obstet Gynecol 136: 179, 1980 |
|
Braun P, Besdine R: Tubo-ovarian abscess with recovery of T-mycoplasma. Am J Obstet Gynecol 117: 861, 1973 |
|
Harwick H J, Purcell RH, Iuppa JB et al: Mycoplasma hominis and abortion. Fertil Steril 33: 551, 1980 |
|
Friberg J: Mycoplasmas and ureaplasmas in infertility and abortion. Fertil Steril 33: 551, 1980 |
|
Wallace RJ, Alpert S. Browne K et al: Isolation of Mycoplasma hominis from blood cultures in patients with postpartum fever. Obstet Gynecol 51: 181, 1978 |
|
McCormack WM, Rosmer B, Lee YH et al: Isolation of genital mycoplasmas from blood obtained shortly after vaginal delivery. Lancet 1: 596, 1975 |
|
Platt R, Lin J-S, Warren JW et al: Infection with Mycoplasma hominis in postpartum fever. Lancet 2: 1217, 1980 |
|
Caspi F, Herczeg E, Solomon F et al: Amnionitis and T-strain mycoplasma. Am J Obstet Gynecol 111: 1102, 1971 |
|
Shurin PA, Alpert S, Rosner B et al: Chorioamnionitis and colonization of the newborn infant with genital mycoplasma. N Engl J Med 293: 5, 1975 |
|
Horne HW, Kundsin RB, Kosasat S: The role of Mycoplasma hominis in human reproductive failure. Fertil Steril 25: 380, 1974 |
|
Braun P, Lee YH, Klein JO et al: Birth weight and genital mycoplasmas in pregnancy. N Engl J Med 284: 167, 1971 |
|
Harrison RF, Hurley R, deLouvois J: Genital mycoplasmas and birth weight in offspring of primigravid women. Am J Obstet Gynecol 133: 201, 1979 |
|
McCormack WM, Braun P, Lee YH et al: The genital mycoplasmas. N Engl J Med 288: 78, 1973 |
|
Zinnemann K, Biberstein EL: Genus Haemophilus. In Buchanan RE, Gibbins NE (eds): Borgey's Manual of Determinative Bacteriology, 8th ed, p 364. Baltimore, Williams & Wilkins, 1974 |
|
Hand WL: Hemophilus species. In Mandell GL, Douglas RG, Bennett JE (eds): Principles and Practice of Infectious Diseases, p 1768. New York, John Wiley & Sons, 1979 |
|
Borchardt KA, Hoke AW: Simplified laboratory technique for diagnosis of chancroid. Arch Dermatol 102:188. 1970 |
|
Hart G: Chancroid, Donovaniasis, lymphogranuloma venereum. Washington, DC, DHEW Publication No. (CDC) 75–8302:1, 1975 |
|
Gaisin A, Heaton CL: Chancroid: Alias the soft chancre. Int J Dermatol 14: 188, 1975 |
|
Asin J: Chancroid: A report of 1,402 cases. Am J Syph Gonor Ven Dis 36: 483, 1952 |
|
Centers for Disease Control: Sexually Transmitted Diseases Treatment Guidelines 1982. MMWR (Suppl) 31:545, 1982 |
|
Hammond G, Lian C, Wilt JC et al: Antimicrobial susceptibility of Haemophilus ducreyi. Antimicrob Agents Chemother 13: 608, 1978 |
|
Hart G: Calymmatobacterium granulomatis. In Mandell GL, Douglas RG, Bennett JE (eds): Principles and Practice of Infectious Diseases, p 1902. New York, John Wiley & Sons, 1979 |
|
Stewart DB: The gynecological lesions of lymphogranu1oma venereum and granuloma inguinale. Med Clin North Am 48: 773, 1964 |
|
Dodson RF, Fritz GS, Hubler WR et al: Donovaniasis: Morphologic study. J Invest Dermatol 62: 611, 1974 |
|
Thew MA, Swift JT, Heaton CL: Ampicillin in the treatment of granuloma inguinale. JAMA 210: 866, 1969 |
|
Maddocks I, Anders EM, Dennis E: Donovanosis in Papua New Guinea. Br J Vener Dis 52: 190, 1976 |
|
Lancefield RC, Hare R: Serologic differentiation of pathogenic and non-pathogenic strains of hemolytic streptococci from parturient women. J Exp Med 61: 335, 1935 |
|
Eickhoff TC, Klein JO, Daly A et al: Neonatal sepsis and other infections due to group B beta-hemolytic streptococci. N Engl J Med 271: 1221, 1964 |
|
Patterson MJ, Hafeez AE: Group B streptococci in human disease. Bacteriol Rev 40: 774, 1976 |
|
Lancefield RC: Microprecipitin-technic for classifying hemolytic streptococci and improved methods for producing antisera. Proc Soc Exp Biol Med 38: 473, 1938 |
|
Baker C J: Summary of the workshop on perinatal infections due to group B streptococcus. J Infect Dis 136: 137, 1977 |
|
Darling CL: Standardization and evaluation of the CAMP reaction for the prompt, presumptive identification of Streptococcus agalactiae (Lancefield group B) in clinical materials. J Clin Microbiol 1: 171, 1975 |
|
Verghese A, Berk SL: Group B streptococcal pneumonia in the elderly. Arch Intern Med 142: 1642, 1982 |
|
Fry RM: Fatal infections of hemolytic streptococcal group B. Lancet 1: 199, 1938 |
|
Finn PD, Holden FA: Observations and comments concerning the isolation of group B beta-hemolytic streptococci from human sources. Can Med Assoc J 103: 249, 1978 |
|
Minkoff HL, Sierra MF, Pringle GF et al: Vaginal colonization with Group B beta-hemolytic streptococcus as a risk factor for post-cesarean section febrile morbidity. Am J Obstet Gynecol 142: 992, 1982 |
|
Faro S: Group B beta-hemolytic streptococci and puerperal infections. Am J Obstet Gynecol 139: 686, 1981 |
|
Pastorek JG, Faro S: Personal observation, 1982 |
|
Baker C J, Clark DJ, Barrett FF: Selective broth medium for isolation of group B streptococci. Appl Microbiol 26: 884, 1973 |
|
Shulman JA: Streptococcus agalactiae (Group B strep-tococcus). In Mandell GL, Douglas RG, Bennett JE (eds): Principles and Practice of Infectious Diseases, p 1607. New York, John Wiley & Sons, 1979 |
|
Steinbrecher UP: Serious infection in an adult due to penicillin-tolerant group B streptococcus. Arch Intern Med 141: 1714, 1981 |
|
Linn DV, Kanarek KS, Pelerson ME: Magnitude of colonization and sepsis by group B streptococci in newborn infants. Curt Microbiol 7: 99, 1982 |
|
Blumberg BS, Alter HJ, Visinich S: A “new” antigen in leukemia sera. JAMA 191: 101, 1965 |
|
King JW: A clinical approach to hepatitis B. JAMA 142: 925, 1982 |
|
Centers for Disease Control: Inactivated hepatitis B virus vaccine. MMWR 31:317, 1982 |
|
Noller KL: Liver disease in pregnancy. In Iffy L, Kaminetzky HA (eds): Principles and Practice of Obstetrics and Perinatology, vol. 2, p 1327. New York, John Wiley & Sons, 1981 |
|
Derso A, Boxall EH, Jarlow MJ et al: Transmission of HBsAg from mother to infant in four ethnic groups. Br Med J 1: 949, 1948 |
|
Shiraki K, Yoshihara N, Kawana T et al: Hepatitis B surface antigen and chronic hepatitis in infants born to asymptomatic carrier mothers. Am J Dis Child 131: 644, 1977 |
|
Czaja AJ, Jurgen L, Baggenstoss AH et al: Corticosteroid-treated chronic active hepatitis in remission. N Engl J Med 304: 5, 1981 |
|
Tam KC, Tai CL, Trepo C et al: Deleterious effect of prednisolone in HBeAg-positive chronic active hepatitis. N Engl J Med 304: 380, 1981 |
|
Seef LB, Wright MPH, Zimmerman HJ et al: Type B hepatitis after needle-stick exposure: Prevention with hepatitis B immune globulin. Ann Intern Med 293: 1055, 1978 |
|
Krugman S: The newly licensed hepatitis B vaccine. JAMA 247: 2012, 1982 |
|
Kumar ML, Nankervis GA: Cytomegalovirus infections. South Med J 72: 854, 1979 |
|
Hanshaw JB: Congenital cytomegalovirus infection: A fifteen year perspective. J Infect Dis 123: 555, 1971 |
|
Ho M: Cytomegalovirus. In Mandell GL, Douglas RG, Bennett JE (eds): Principles and Practice of Infectious Diseases, vol. 2, p 1307. New York, John Wiley & Sons, 1979 |
|
Lennette EH, Schmidt NJ: Diagnostic Procedures for Viral and Rickettsial Infections. New York. American Public Health Association, 1969 |
|
Rao N, Waruszewski DT, Ho M et al: Evaluation of the anticomplement immunofluorescence test in cytomegalovirus infection. J Clin Microbiol 6: 633, 1977 |
|
Betts RF, George SD, Rundell BR et al: Comparative activity of immunofluorescent antibody and complement-fixing antibody in cytomegalovirus infection. J Clin Microbiol 4: 151, 1976 |
|
Leinikki P, Granstrom ML, Santavaori P et al: Epidemiology of cytomegalovirus infections during pregnancy and infancy. Scand J Infect Dis 10: 165, 1978 |
|
Drew WL, Miner RC: Transfusion-related cytomegalovirus infection following non-cardiac surgery. JAMA 247: 2389, 1982 |
|
Sargal S, Lunyk O, Larke RPB et al: The outcome in children with congenital cytomegalovirus infection. Am J Dis Child 136: 902, 1982 |
|
Stagno S, Pass RF, Reynolds DW et al: Comparative study of diagnostic procedures for congenital cytomega1ovirus infection. Pediatrics 65: 251, 1980 |
|
Gehrz RC, Christianson WR, Linnet KM et al: Cytome-galovirus vaccine. Arch Intern Med 140: 936, 1980 |
|
Fleisher GR, Start SE, Friedman HM et al: Vaccination of pediatric nurses with live attenuated cytomegalovirus. Am J Dis Child 136: 194, 1982 |
|
Armstrong D: Listeria monocytogenes. In Mandell GL Douglas RG, Bennett JE (eds): Principles and Practice of Infectious Diseases, vol. 2, p 1626. New York, John Wiley & Sons, 1979 |
|
Gray ML, Killinger AH: Listeria monocytogenes and listeric infections. Bact Rev 30: 309, 1966 |
|
Busch LA: Human listeriosis in the United States, 1967-1969. J Infect Dis 123: 328, 1971 |
|
Bojsen-Moller J: Human listeriosis: Diagnostic, epidemiological and clinical studies. Acta Pathol Microbiol Stand (Suppl) 229: 1, 1972 |
|
Holshouser CA, Ansbacher R, McNitt T et al: Bacterial endocarditis due to Listeria monocytogenes in a pregnant diabetic. Obstet Gynecol 51: 9S, 1978 |
|
Katz VL, Weinstein L: Antepartum Treatment of Listeria monocytogenes septicemia. South Med J 75: 1353, 1982 |
|
Jones TC: Entatmoeba histolytica (amebiasis). In Mandell GL, Douglas RG, Bennett JE (eds): Principles and Practice of Infectious Diseases, vol. 2, p 2087. New York, John Wiley & Sons, 1979 |
|
DuPont HL: Shigella species (bacillary dysentery). In Mandell GL, Douglas RG, Bennett JE (eds): Principles and Practice of Infectious Diseases, vol. 2, p 1751. New York, John Wiley & Sons, 1979 |
|
Weinstein BB, Week JC: Amebic vaginitis. Am J Obstet Gynecol 56: 180, 1948 |
|
Hingorani V, Mahapatra LN: Amebiasis of vagina and cervix. J Int Coil Surg 42: 662, 1964 |
|
Grigsby WP: Surgical treatment of amebiasis. Surg Gynecol Obstet 128: 609, 1969 |
|
Amstey MS, Candell DL: Salpingitis-perihepatitis in a patient with cervical Shigella sonnei. Obstet Gynecol 55 (Suppl): 70S, 1980 |
|
Mungiua H, Franco E, Valenzuela P: Diagnosis of genital amebiasis in women by the standard Papanicolaou technique. Am J Obstet Gynecol 94: 181, 1966 |
|
Mahmoud AAF: Current concepts: Schistosomiasis. N Engl J Med 297: 1329, 1977 |
|
Mahmoud AAF: Trematodes (schistosomiasis, flukes). In Mandell GL, Douglas RG, Bennett JE (eds): Principles and Practice of Infectious Diseases, vol. 2, p 2173. New York, John Wiley & Sons, 1979 |
|
Williams AO: Pathology of schistosomiasis of the uterine cervix due to S haematobium. Am J Obstet Gynecol 98: 784, 1967 |
|
Berry A: A cytopathological and histopathological study of bilharziasis of the female genital tract. J Pathol Bact 91: 325, 1966 |
|
Shafeek MA, Osman MI, Mohamed HM et al: The association of schistosomiasis with female genital malignancies. Tenth World Congress of Gynecology and Obstetics, San Francisco, October 1982 (abstr #1707) |
|
Osman HE, Shafee MA, Hammond MM et al: The value of vaginal and cervical cytology in the diagnosis of genital schistosomiasis, Tenth World Congress of Gynecology and Obstetrics, San Francisco, October 1982 (abstr # 1708 ) |
|
Fields BT: Blastomyces dermatitidis. In Mandell GL, Douglas RG, Bennett JE (eds): Principles and Practice of Infectious Diseases, vol. 2, p 2047. New York, John Wiley & Sons, 1979 |
|
Stevens DA: Coccidioides immitis. In Mandell GL, Douglas RG, Bennett JE (eds): Principles and Practice of Infectious Disease, vol. 2, p. 2053. New York, John Wiley & Sons, 1979 |
|
Lerner PI: Actinomyces and Arachnia species. In Mandell GL, Douglas RG, Bennett JE (eds), Principles and Practice of Infectious Diseases, vol. 2, p 1969, New York, John Wiley & Sons, 1979 |
|
Davis MIJ: Analysis of 46 cases of actinomycosis with special reference to its etiology. Am J Surg 52: 447, 1941 |
|
Valicenti JF, Pappa AA, Graberc D et al: Detection and prevalence of IUD-associated Actinomyces colonization and related morbidity, JAMA 247:1149, 1982 |
|
Burkman R, Schlesselman S: The relationship of genital tract actinomycetes and the development of pelvic inflammatory disease. Am J Obstet Gynecol 143: 585, 1982 |
|
Braby H, Dougherty CM, Mickal A: Actinomycosis of the female genital tract. Obstet Gynecol 23: 580, 1964 |
|
Persson EA, Holmberg K: Serologic test for genital actinomycosis. Tenth World Congress of Gynecology and Obstetrics, San Francisco, October 1982 (abstr #1717) |
|
Bacterial infection from actinomyces, though rare, is found to be a problem among some IUD users. Fam Plann Perspect 12:306, 1980 |